News
Can diamonds produce methane?
16.12.2021

It doesn’t seem like a good idea to turn one of the world’s most beautiful gems — diamonds — into one of the worst greenhouse gases — methane. But a group of researchers from the Universities of Bologna and Edinburgh (United Kingdom), the Centre National de la Recherche Scientifique (France) and HPSTAR (China) have succeeded. This result, published in the journal Nature Communications, was not a clumsy laboratory error. Rather, this study could shed more light on the deep carbon cycle and the formation of hydrocarbons by abiotic processes (i.e., unrelated to biological activities) deep in the Earth.
The deep Earth carbon cycle accounts for about 90% of the total carbon cycle. Despite this, the cycle that occurs below the Earth’s surface has been poorly understood. This phenomenon is critical to life on our planet because it allows carbon deep in the Earth to return to the atmosphere.
“It is well known that the decomposition of methane can lead to the formation of diamonds. What has been less well known is that the reverse process is also possible. The methane produced by the reaction between diamonds and hydrogen has been the missing element for a more complete understanding of the deep carbon cycle,” explains Alberto Vitale Brovarone, professor at the Department of Biology, Geology and Environmental Sciences at the University of Bologna and also one of the study’s authors.
The deep carbon cycle also includes the formation of hydrocarbons such as methane as a result of processes that do not involve biological activities. This theory has been debated for more than a century. To test this theory, the researchers started with diamonds, which are essentially gems in the Earth’s mantle consisting of solid carbon atoms in a crystal structure.
The scientists used a “diamond anvil cell,” a high-pressure experimental apparatus that presses two diamond spheres against each other to replicate the pressure conditions of the Earth’s upper mantle at a depth of more than 70 km. The researchers then pushed an atmosphere of pure hydrogen at 300 °C and watched as methane, whose molecules are composed of one carbon and four hydrogen atoms (CH4), rapidly formed.
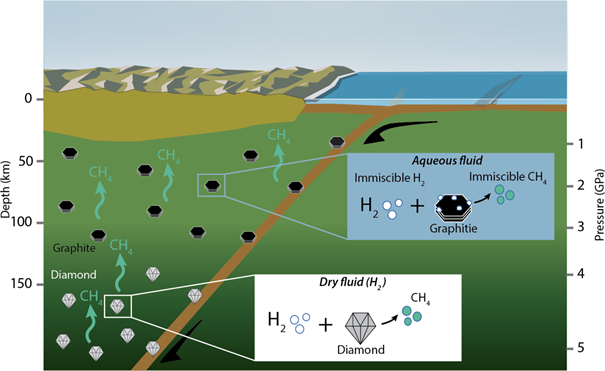
The researchers repeated this experiment by adding graphite, which is also pure carbon, and a glassy carbon material. In both cases, they observed that methane formed faster and in greater quantities than when they used only diamonds. These results suggest that carbon-based graphitic materials could be very efficient reagents and therefore could serve as energy sources for the methane reserves in the Earth’s upper mantle.